Mechanism of PML Nuclear Bodies biogenesis & oxidative stress response
PML nuclear bodies (PML NBs) are stress-responsive compartments organized by the scaffolding ProMyelocytic Leukemia protein (PML) (Lallemand-Breitenbach and de The, 2018). PML polymerizes into the spherical outer shell of the bodies and recruits a variety of unrelated partner proteins within their inner core. PML NBs have been involved in various biological processes such as senescence, apoptosis, self-renewal and more recently metabolism, raising the question of any unifying function underlying these diverse activities. Critically, PML NBs also play a key role in the therapeutic response of hematological malignancies, notably Acute Promyelocytic Leukemia (APL, see below).
From a cell biology perspective, we have dissected the biochemical assembly of PML NBs. The latter is initiated by interactions of the multiple self-dimerizing domains of PML (RING, coiled coil, B2 box...). PML is a cysteine-rich and oxidation-prone protein. While some of these cysteines participate in Zn-finger domain folding, others may undergo oxidation (Jeanne et al., 2010). We recently unraveled the molecular basis for the ROS-dependency of PML NB formation. The B2 box may undergo trimer formation (Bercier et al., 2023) through a highly conserved a-helix, which is mutated in therapy-resistant APL patients (Lehmann-Che et al., 2014; Lehmann-Che et al., 2018). This trimeric arrangement exposes a critical cysteine residue, C213, which forms a ROS-reactive trio that controls PML NBs assembly dynamics (Bercier et al., 2023). Importantly, arsenic trioxide, an efficient APL therapy binds this cysteine trio to dramatically affect NB-biogenesis, the first steps of its therapeutic efficacy.

SUMOs are ubiquitin-like polypeptides mainly involved in protein complex stabilization, through their ability to interact with SUMO-interacting Motifs (SIMs). Target protein sumoylation is regulating multiple processes including DNA repair, transcription control... Sumoylation relies on a single E2 conjugating enzyme, UBC9, which is efficiently recruited by PML NBs, likely explaining the unusually efficient of PML sumoylation (Sahin et al., 2014; Wang et al., 2018). PML sumoylation on K160 subsequently controls the recruitment of partner proteins through their SIMs (Lallemand-Breitenbach et al., 2001; Sahin et al., 2014). Remarkably, in mESC, the effects of Pml loss or Ubc9 loss are highly similar, suggesting that a key function of PML is indeed to favor UBC9-mediated partner SUMO-conjugation (Tessier et al., 2022). Altogether, this ROS-sensing mechanism couples subcellular organization to sumoylation control through PML NB formation. Pml-/- mice, which are devoid of NBs, develop normally and live well, yet, their redox signaling is profoundly altered (Niwa-Kawakita et al., 2017).
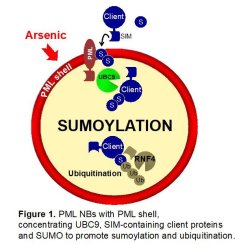
While investigating arsenic-induced degradation of PML/RARA in APL (see below), we unravelled a proteolysis pathway triggered by sequential SUMO and ubiquitin conjugation, thanks to the SIM-containing ubiquitin ligase RNF4 (Lallemand-Breitenbach et al., 2008). While initially described for PML and PML/RARA, this pathway was later shown to degrade many other partner proteins. Thus, PML NBs are hubs controlling sumoylation and degradation of a broad variety of proteins in response to oxidative stresses in vivo.
Harnessing PML Nuclear Bodies for leukemia therapy
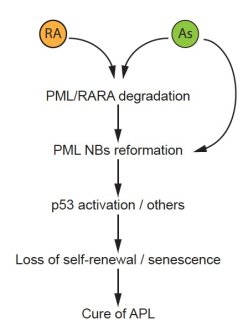
PML NBs gained considerable interest after our observation that they were disrupted in a treatment-reversible manner APL (Daniel et al. Blood 1993). APL is caused by the oncogenic PML/RARA fusion protein. The two clinically efficient drugs, all trans-retinoic acid (RA) and arsenic trioxide (As) directly target PML/RARA for degradation, through well-characterized proteolytic pathways (de The et al., 2017). Importantly, we have demonstrated that PML and PML NB reformation are required for APL response to therapy in murine APL models (Ablain et al., 2014). The key role of PML nuclear bodies in the cure of APL patients was confirmed by the discovery of PML mutations in the rare therapy-resistant patients (Lehmann-Che et al., 2014). Arsenic-induced NB reorganization synergizes with therapy-induced PML/RARA degradation to trigger APL cure (Ablain et al., 2014). Therapeutically, we unraveled highly synergistic effects of RA/arsenic combination in APL mouse model (Lallemand-Breitenbach et al., 1999), a regimen that was successfully transferred to clinic, so that most APL patients are now definitively cured with this combination (Lo-Coco et al., 2013, de Thé, 2017, 2018).
In recent studies, we have generalized the importance of enforcement of PML NB formation and downstream signaling in other malignancies, notably those where PML NBs are abnormal (Dagher et al., 2021; Wu et al., 2021). Thus, the curative pathways that we have outlined in APL may be shared by other therapy/malignancy couples and emerge as new therapeutic targets (Rerolle and de The, 2023). Ongoing studies are exploring the molecular bases for leukemia response to conventional chemotherapy in mice and in patients.

