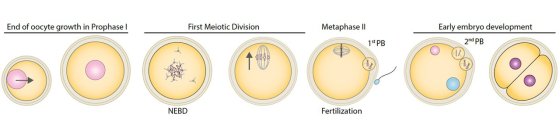
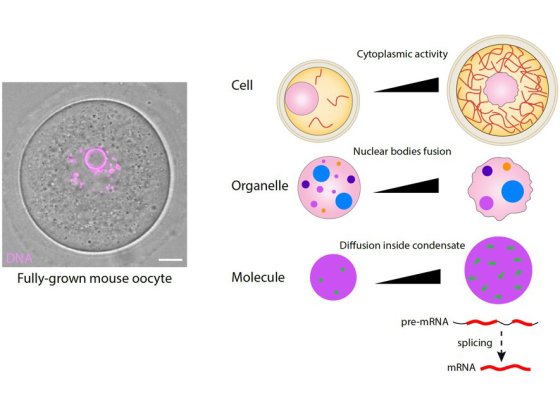
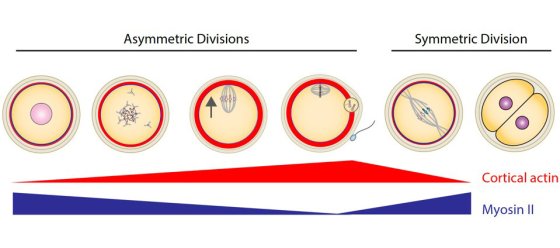
At fertilization, the sperm mostly transmit their genome but oocytes are transmitted in full to the next generation. Using cell biology, genetics, computational biology and biophysics approaches of meiotic divisions, we study the nature of this maternal inheritance, its transmission, and the consequences when it goes awry.
In the human species, female gamete production is error-prone, generating a high basal rate of poor-quality oocytes (10-20%) increasing with the mother's age, leading to infertility, miscarriage and congenital syndromes such as trisomies. This is a public health problem in our modern world, where women tend to delay the age of their first pregnancy, reflecting societal changes. A direct consequence is that oocyte freezing for fertility preservation and the use of ART (Assisted Reproductive Technologies) increases worldwide, yet only 20% of ART cycles result in a birth. Gamete quality is a key parameter for it success rate. However, contrary to sperm, no biomarker exists to evaluate oocyte quality in ART. An important fundamental question is therefore to define what oocyte quality is, in order to be able to recognize it. In other words, what is the nature of the information contained in the oocyte that shapes its quality and enables the formation of a new individual? While the sperm mostly transmit their genome at fertilization, oocytes are transmitted in full to the next generation, suggesting that oocyte quality is determined not only by its genome, but also by its large cytoplasm. To explore this maternal inheritance, we study the last stages of mammalian oogenesis, namely the end of oocyte growth followed by meiotic divisions, and early embryonic development (Fig 1).