Résumé
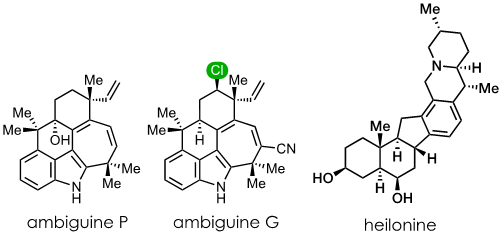
The process of chemical synthesis involves a sequence of reactions that systematically elevate the complexity of a starting material until it ultimately morphs into the desired end product. While the specific reactions – or tactics – employed for each step are undeniably crucial for success, the underlying strategy is arguably of greater importance. The strategy should, ideally, not only address the primary structural challenges of a natural product, but it should also enable the synthesis of other members of that family of natural products. In this presentation, I will delve into the strategic and tactical considerations that shaped our work on the synthesis of indole alkaloid metabolites derived from cyanobacteria. Particular emphasis will be placed on the pentacyclic ambiguine group of compounds, which form a subset of the large hapalindole family of alkaloids. Time permitting, I will also discuss our approach for the synthesis of heilonine, a member of the Veratrum family of alkaloids.
Références
Comprehensive review: Bhat, V., Dave, A., MacKay, J.A., Rawal, V.H. The Chemistry of Hapalindoles, Fischerindoles, Ambiguines, and Welwitindolinones, The Alkaloids: Chemistry and Biology, 2014, 73, 65-160. DOI: https://doi.org/10.1016/B978-0-12-411565-1.00002-0
Ambiguine P: Xu, J.; Rawal, V.H. Total Synthesis of (-)-Ambiguine P, J. Am. Chem. Soc. 2019, 141, 4820-4823. DOI: https://doi.org/10.1021/jacs.9b01739
Ambiguine G: Hu, L. B.; Rawal, V. H. Total Synthesis of the Chlorinated Pentacyclic Indole Alkaloid (+)-Ambiguine G, J. Am. Chem. Soc. 2021, 143, 10872–10875. DOI: https://doi.org/10.1021/jacs.1c05762
Cassaidy, K. J.; Rawal, V. H. Enantioselective Total Synthesis of (+)-Heilonine, J. Am. Chem. Soc. 2021, 143, 16394–16400. DOI: https://doi.org/10.1021/jacs.1c08756
